News
Home News Technical articles Sterile filtration products used in monoclonal antibody technology
Home News Technical articles Sterile filtration products used in monoclonal antibody technology
Sterile filtration products used in monoclonal antibody technology
2024-03-04 Eternalwater Monoclonal antibodies have the advantages of uniform structure, high purity, strong specificity, high potency, few cross-reactions, and low preparation costs. They have become one of the fastest growing fields in the biopharmaceutical industry and have been successfully used to treat a variety of cancers, Autoimmune diseases and other diseases play an important role in biomedicine. This article gives a brief introduction to the relevant knowledge and filtration solutions of monoclonal antibodies.About monoclonal antibodies
1.Antigen
Before introducing antibodies, let us first understand what an antigen is? Antigen refers to something that can stimulate the body to produce a (specific) immune response, and can combine with immune response product antibodies and sensitized lymphocytes in vitro to produce immunity.
2.Antibody
Antibodies refer to immunoglobulins produced by plasma cells differentiated from B lymphocytes by the body's immune system under the stimulation of antigens, and can specifically bind to the corresponding antigens.
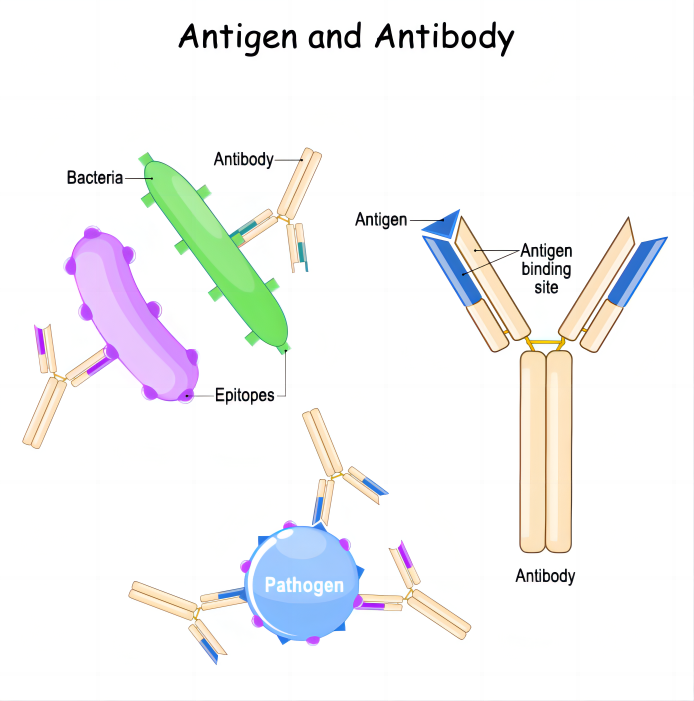
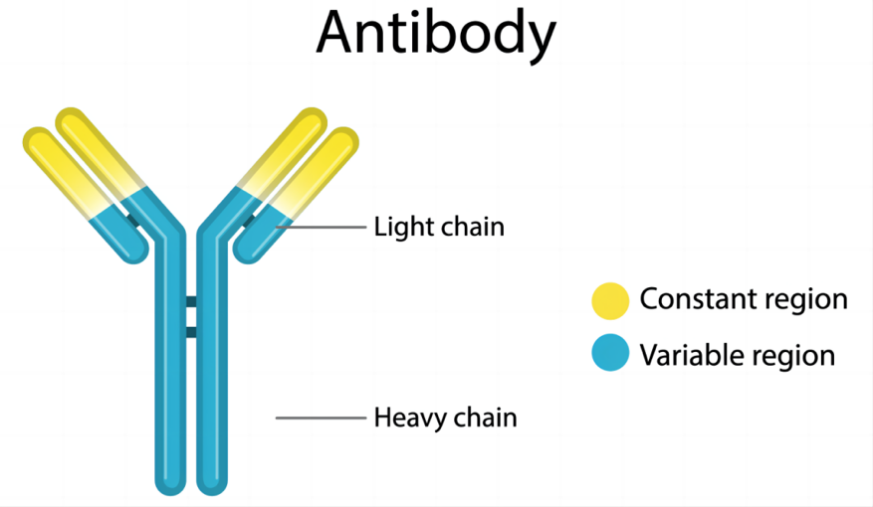
Monoclonal antibodies ( mAbs ) are highly uniform antibodies produced by B cells that only target a specific antigenic epitope. A "Y" shaped immunoglobulin used to identify and eliminate foreign substances such as bacteria and viruses. Typically, natural antibodies are composed of two heavy chains (H chains) and two light chains (L chains). The heavy chain is longer and the relative molecular weight is larger; the light chain is shorter and the relative molecular weight is smaller; the chains are connected by disulfide bonds and non-covalent bonds to form a monomer molecule. The entire antibody can be further divided into two parts : the constant region (C region) and the variable region (V region).
Techniques in Monoclonal Antibody Preparation
In 1975, British scientists Kohler and Milstein first combined long-lived mouse myeloma cells that cannot produce specific antibodies with short-lived plasma cells (mouse splenocytes immunized with sheep red blood cells) that can produce specific antibodies after immunization. Fusion in vitro creates hybridoma technology. Thanks to this technology, mAbs can be prepared in large quantities for basic research and have clinical translation capabilities, opening a new era in the development of monoclonal antibody drugs.
The figure below shows the classic preparation method of monoclonal antibodies. Specific antigens are injected into mice to trigger an immune response, and corresponding B lymphocytes are obtained, which are fused with mouse myeloma cells. After screening, the obtained hybridoma cells are Large-scale culture in vitro to amplify monoclonal antibodies.
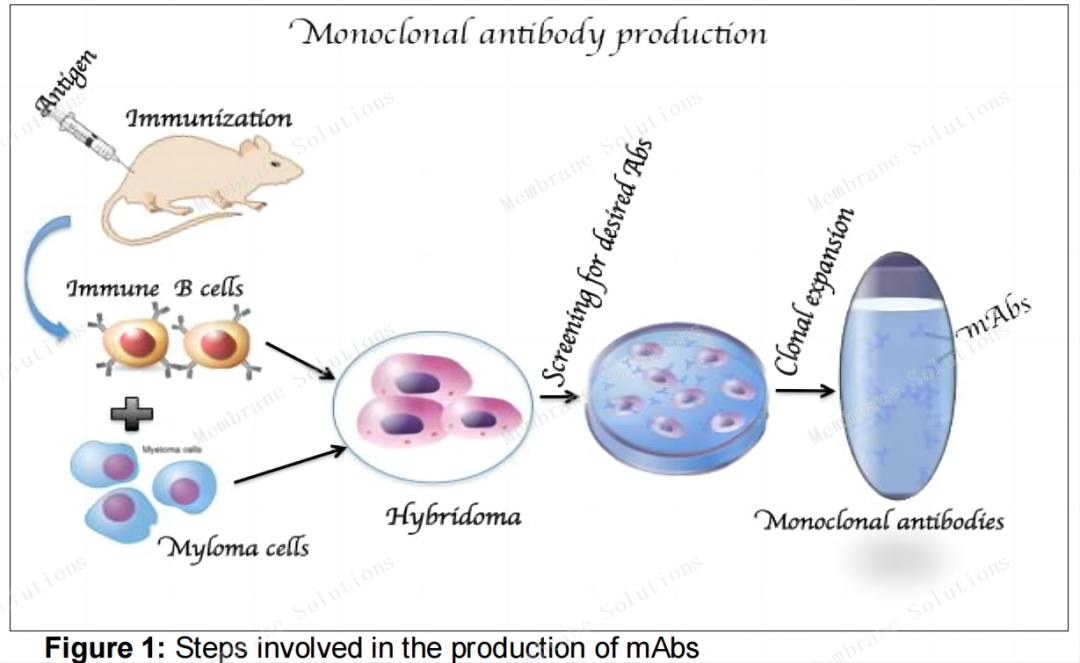
Four stages of monoclonal antibody development
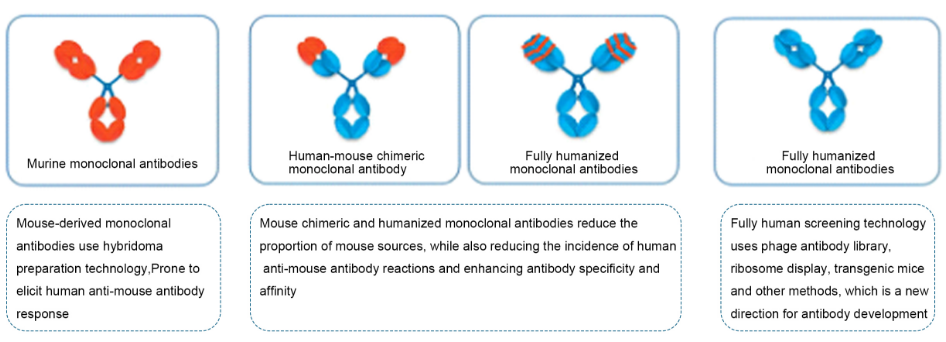
The development of monoclonal antibodies has mainly gone through four stages: murine monoclonal antibodies, human-mouse chimeric monoclonal antibodies, humanized monoclonal antibodies and fully human monoclonal antibodies. Since mouse-derived antibodies will always produce adverse reactions of one kind or another when applied to humans, humanized antibodies are the development trend of future drugs.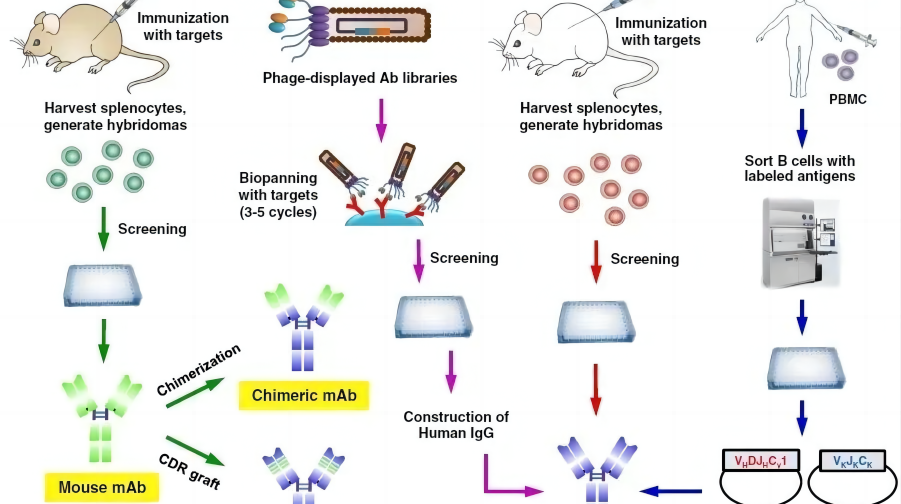
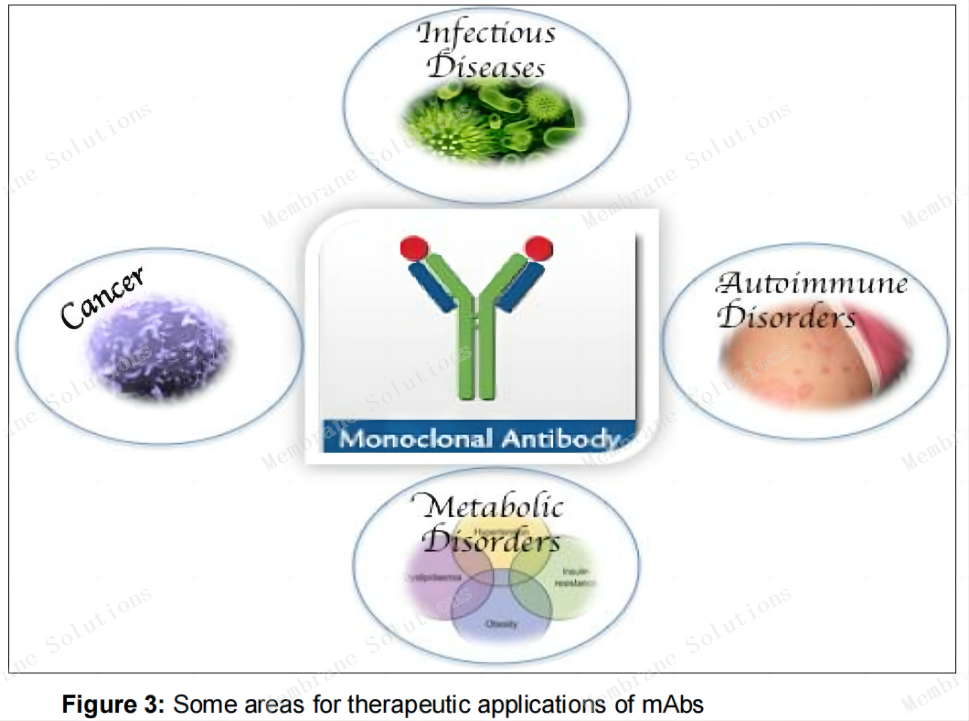
Applications of monoclonal antibodies
Laboratory medicine diagnostic reagents: widely used in enzyme-linked immunosorbent assay, radioimmunoassay, immunohistochemistry, flow cytometry and other technologies. And the application of monoclonal antibodies has greatly promoted the development of commercial kits, which are widely used for the detection of antigens and antibodies, hormones, cytokines, etc.
Protein purification: As an important ligand in affinity chromatography, it is prepared into a chromatography column to achieve separation and purification of proteins.
high flow filter cartridge
Tumor-directed treatment and radioimmunoassay technology: using the guiding effect of monoclonal antibodies, connecting drugs and monoclonal antibodies to guide target cells and directly killing target cells; and linking radioactive markers to monoclonal antibodies for radioimmunoassay imaging.
Filtration Solutions for Monoclonal Antibody Processing
The production process stages of monoclonal antibodies mainly include the upstream cell culture process, the harvesting and clarification process of cell culture fluid, and the downstream separation and purification process . The cell culture fluid containing antibodies obtained after the upstream cell culture is completed will contain contaminants such as proteases, host cells, cell debris, host cell proteins, host cell nucleic acids, and components of the culture medium and feed solution, which will affect the stability and purity of the antibodies. , it needs to be removed to clarify the cell fluid before it can be used in the subsequent antibody separation and purification process; the main purpose of downstream separation and purification is to separate the antibody from process-related impurities and product-related impurities, and finally obtain high-purity, low-potentially harmful antibody drugs .Upstream cell culture process:
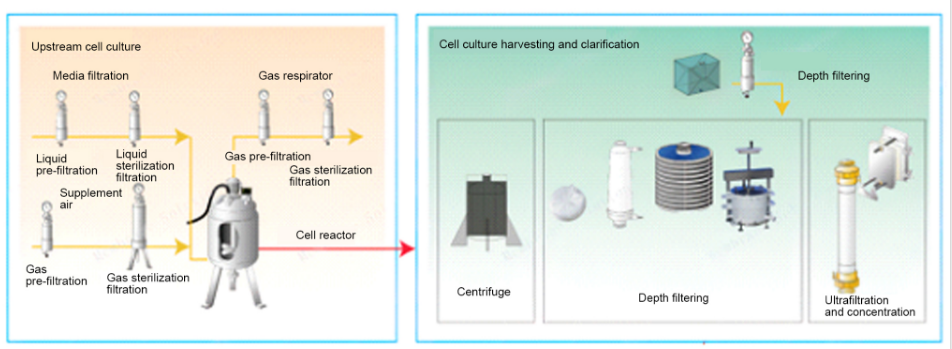
Downstream process - three-step chromatography:
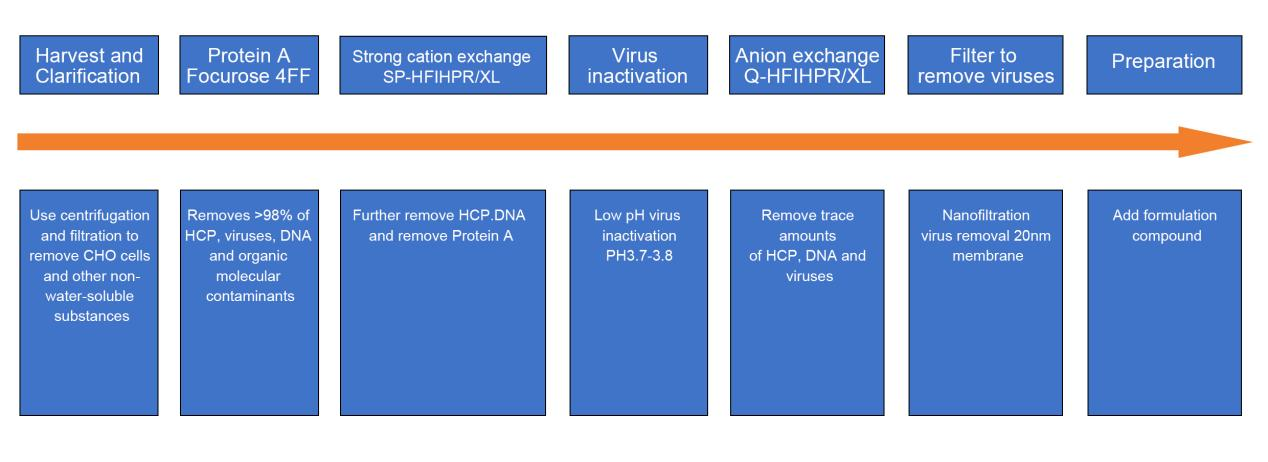
Among them , filtration is widely used in different process stages, mainly including sterilizing filtration of culture medium, sterilizing filtration of gas, sterilizing filtration of buffer, clarifying filtration of dead cells and cell debris, ultrafiltration concentration, Chromatography purification, sterilization filtration of end products, virus removal filtration, etc.
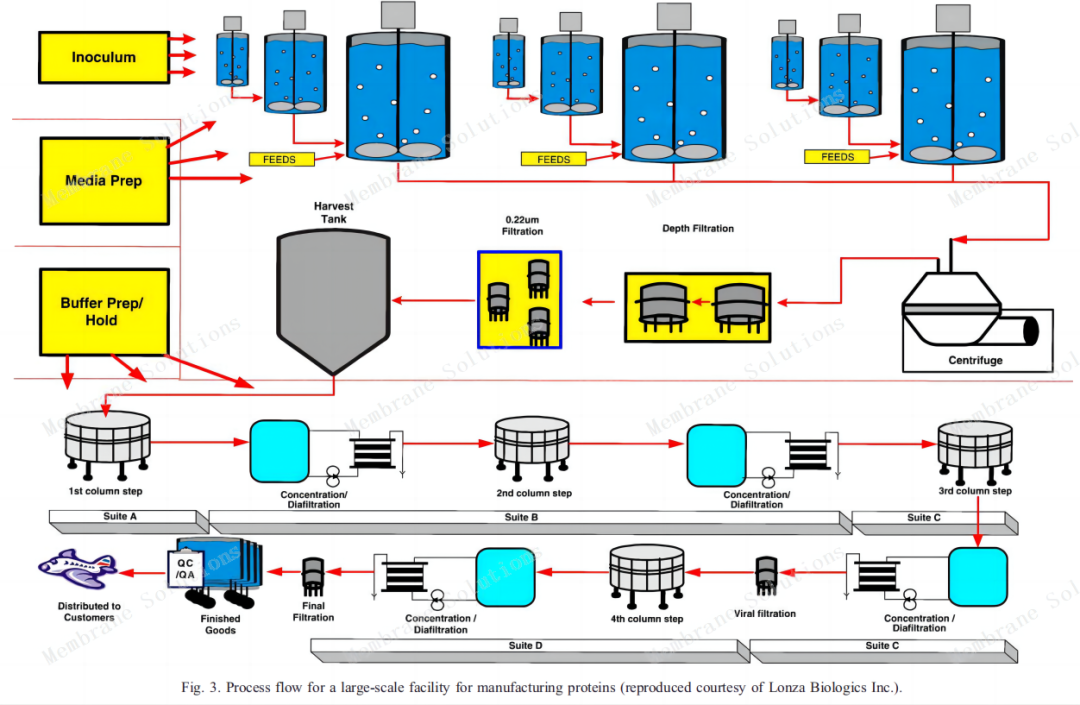
20000L production process:
Traditional monoclonal antibody preparation process flow chart:
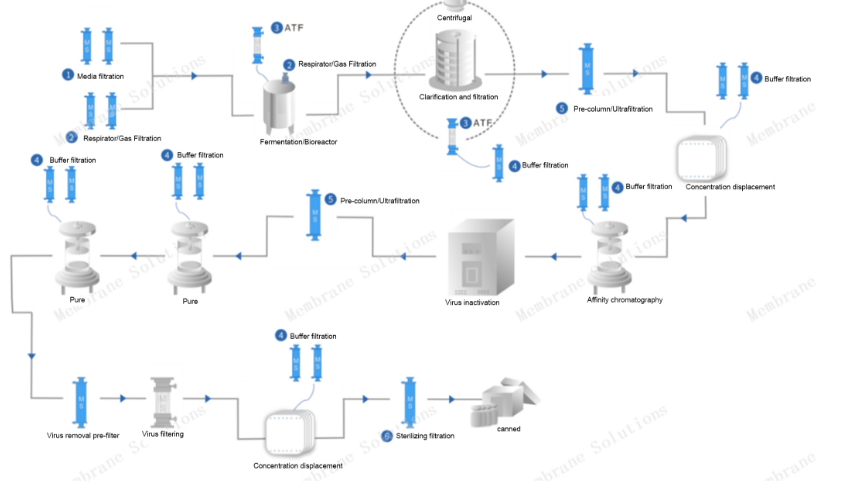
Its main filtering sites are:
1. Media filtration: remove bacteria and microorganisms in cell culture media;2. Respirator/gas filtration: Gas sterilization filtration is mainly used in the air inlet and exhaust filtration of bioreactors, respirators in various storage tanks and batching tanks, etc., mainly to remove bacteria and other microorganisms in the gas;
3.ATF: Tangential flow filtration to remove dead cells and cell debris as well as lipids, colloids, particles and other impurities;
4. Buffer filtration: sterilizing filtration of buffer to extend the service life of chromatography columns and ultra-filtration membranes;
5. Security filtration: pre-filtration before ultra-filtration to protect the ultra-filtration module;
6. Terminal sterilization filtration: remove bacteria and microorganisms in the terminal filtrate.
Latest News
Read more
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
CopyRight © Hangzhou Eternalwater Filtration Equipment Co., Ltd. 2002-2025
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN