Home News Technical articles Three-Step Method for Troubleshooting the Failed Integrity Test of Sterilizing Filter Cartridge
Three-Step Method for Troubleshooting the Failed Integrity Test of Sterilizing Filter Cartridge
2025-12-23 AdminIn sterile drug production, microbial removal from liquids or gases is typically achieved through terminal heat sterilization or physical retention methods. The physical retention approach involves sterilizing filtration to meet the relevant quality requirements for sterile drugs. Filter integrity is one of the key control points in the confirmation and release of sterile drug production. Therefore, post-use integrity test of filters is an essential operation in the production process, and companies similarly perform pre-use integrity test of filters.
In pharmaceutical production, sterilizing filter integrity test is a critical step in ensuring sterility assurance. If a sterilizing filter fails the integrity test, it may be damaged, but there could also be other potential causes of failure.
Integrity Test Methods:
1. Bubble Point Test Principle:
A fully wetted filter membrane requires sufficient gas pressure to overcome surface tension and displace liquid from the pores. This pressure is defined as the Bubble Point Pressure. For a fully wetted membrane, the bubble point pressure is inversely proportional to the pore size; meaning a smaller pore size corresponds to a higher bubble point. The bubble point value is not affected by the membrane area.
2. Forward Flow Test Principle:
The filter is fully wetted and subjected to a gas pressure lower than the bubble point pressure, resulting in a small gas flow on the downstream side. This flow occurs due to the "diffusion" of gas through the membrane. A larger filter membrane area results in a higher diffusive flow value.
3. Water Intrusion Test Principle:
Hydrophobic filter membranes prevent water passage at low pressures. At a pressure below the water breakthrough pressure (where water is forced through), a small but measurable flow of water occurs, which is essentially the evaporation flow that is detected. the appropriate test parameters are used, and the proper wetting fluid and wetting procedure are followed.
This article details the troubleshooting methods for the failed integrity test of sterilizing filter cartridge :
Step 1:
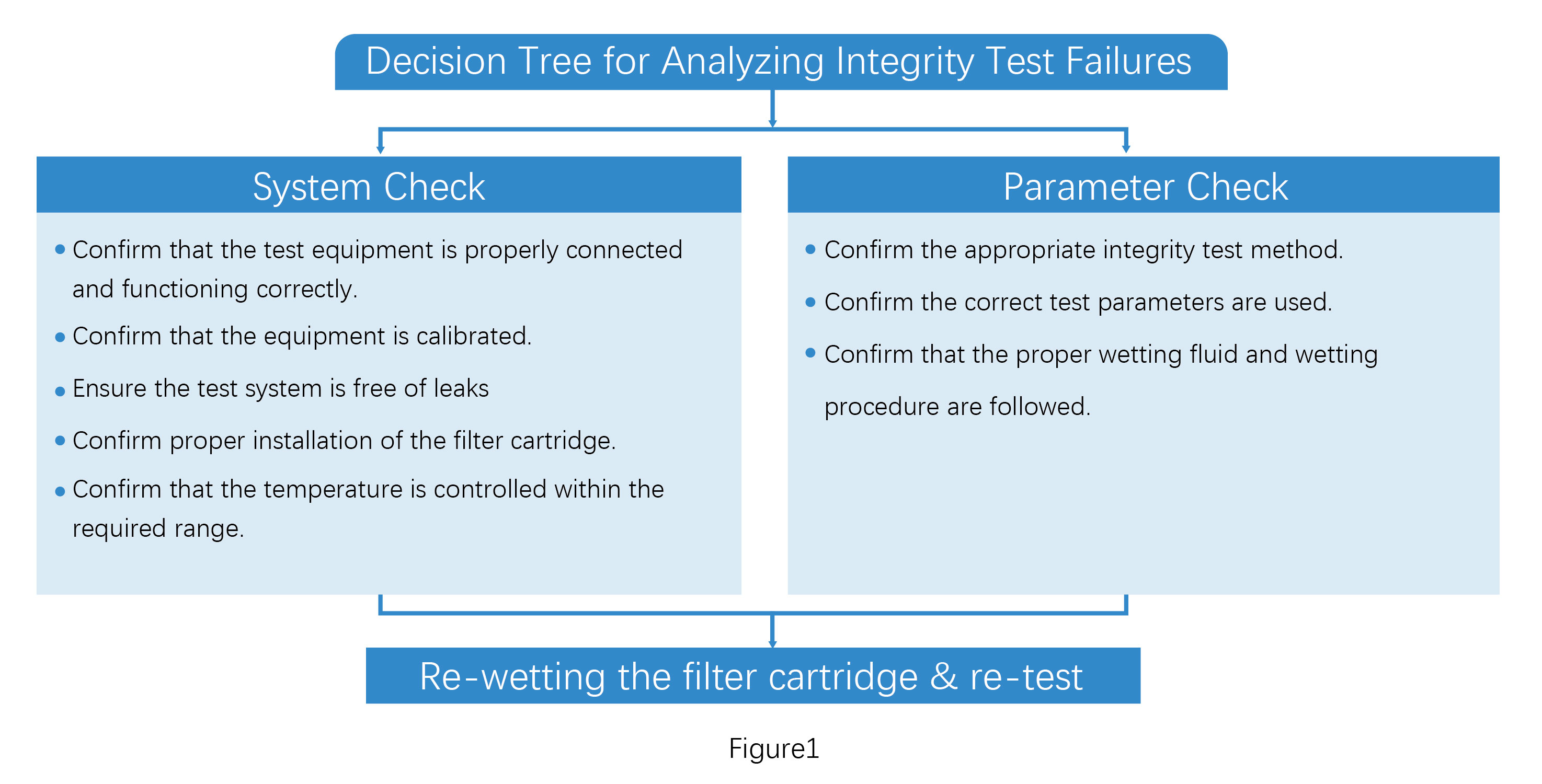
System Check:Confirm the test equipment connections and calibration status, check the system for leaks, ensure proper filter cartridge installation, and confirm that the ambient temperature is within the required range.
Parameter Check:
Confirm that the correct integrity test method is selected, the appropriate test parameters are used, and the proper wetting fluid and wetting procedure are followed.
Proceed with troubleshooting according to Figure 1. If no issues are identified in the system and parameter checks, re-wet the filter cartridge and re-conduct the integrity test.
Step 2:
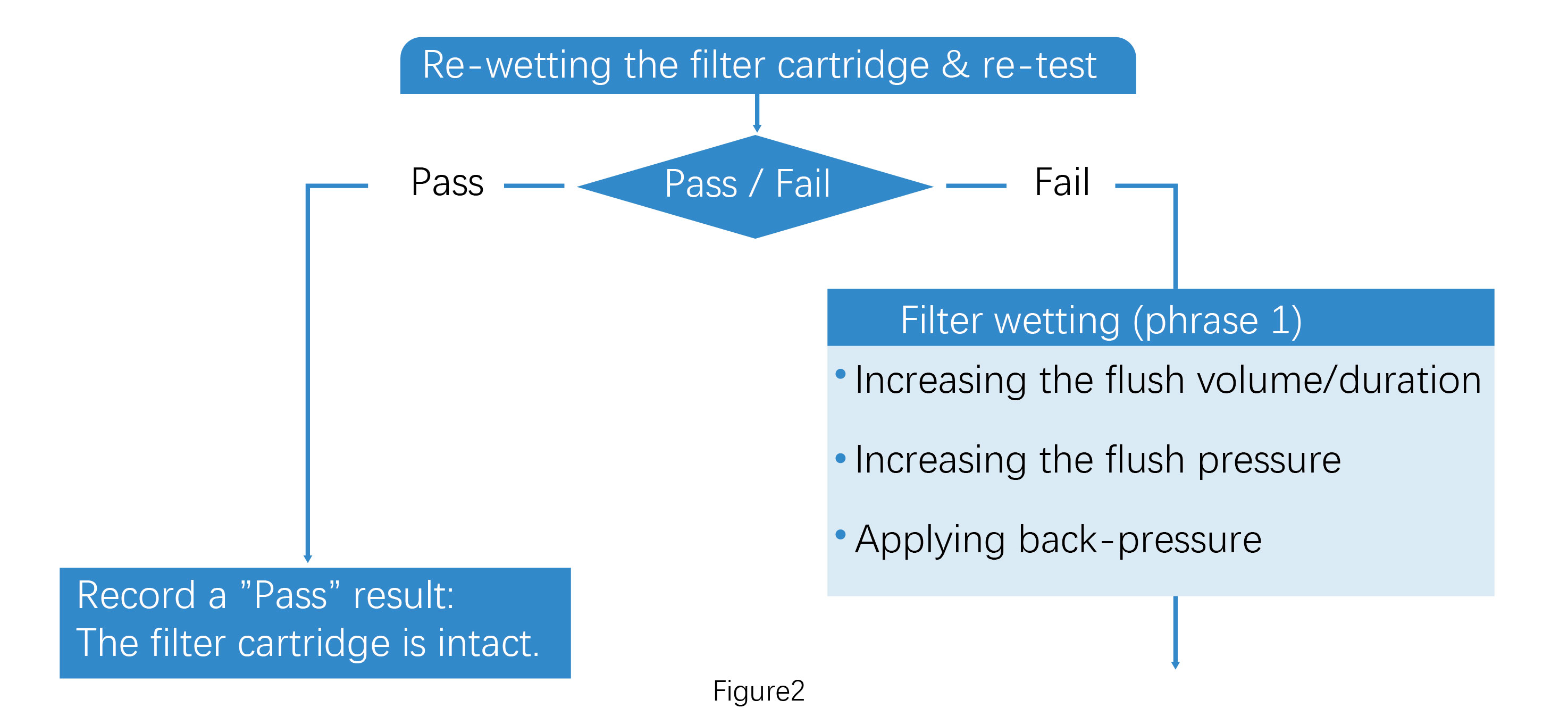
After following the troubleshooting steps in Figure 1, if the integrity test passes, record the filter cartridge as intact. If it fails, proceed with Figure 2. In this stage, further wetting of the filter cartridge can be attempted by increasing the flush volume/duration, increasing the flush pressure and applying back-pressure (Wetting Phase 1).
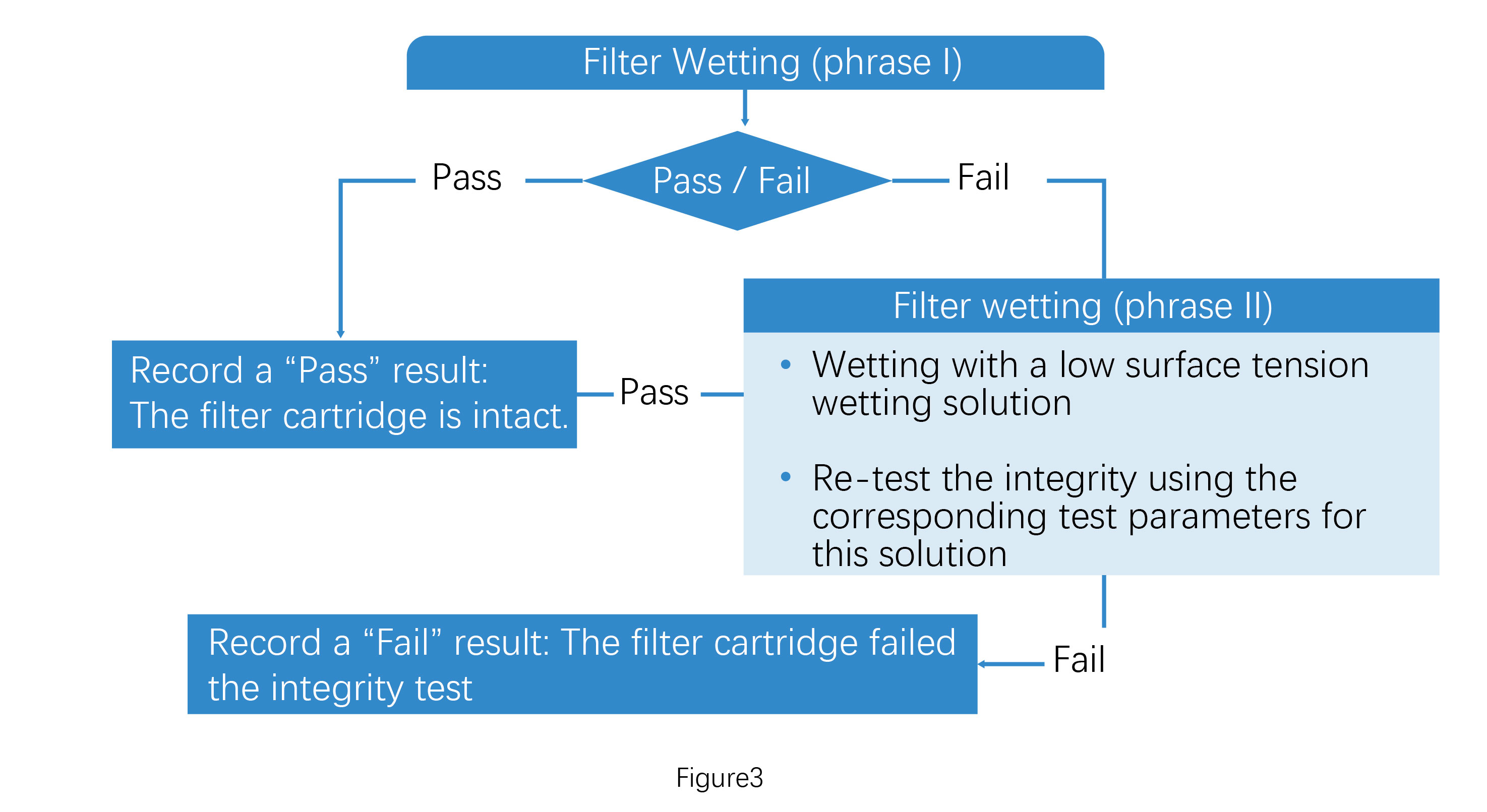
Step 3:
After following the troubleshooting steps in Figure 2, perform the integrity test on the filter cartridge that has undergone Wetting Phase I. If the test passes, record the filter cartridge as intact.. If the test fails, proceed to Figure 3. Continue with Wetting Phase II, which involves using a low surface tension wetting solution (primarily ethanol or isopropanol) to enhance wetting. Re-test the integrity using the corresponding test parameters for this solution. If this subsequent test passes, record the filter as intact. If this test fails, record a failure of the filter integrity test.
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN