Home News Technical articles Application of sterilizing filtration products in injection production process
Application of sterilizing filtration products in injection production process
2024-03-12 EternalwaterInjections refer to raw materials or sterile preparations made with appropriate excipients for injection into the body. Injections are divided into large-volume injections (large infusion or LVP), small-volume injections (small injections or SVP) and powder injections according to their production technology characteristics and production processes. Among them: large-volume injection (LVP) is commonly known as large infusion, which refers to a volume of ≥ 50ml liquid sterilization preparation that is directly injected into the vein; small volume injection (SVP), commonly known as water injection, configures the drug into a solution (aqueous or non-aqueous), suspension or emulsion, and puts it into an ampoule or multi-dose container. The prepared sterile preparation has a general capacity of ≤20mL.
membrane pleated filter cartridge
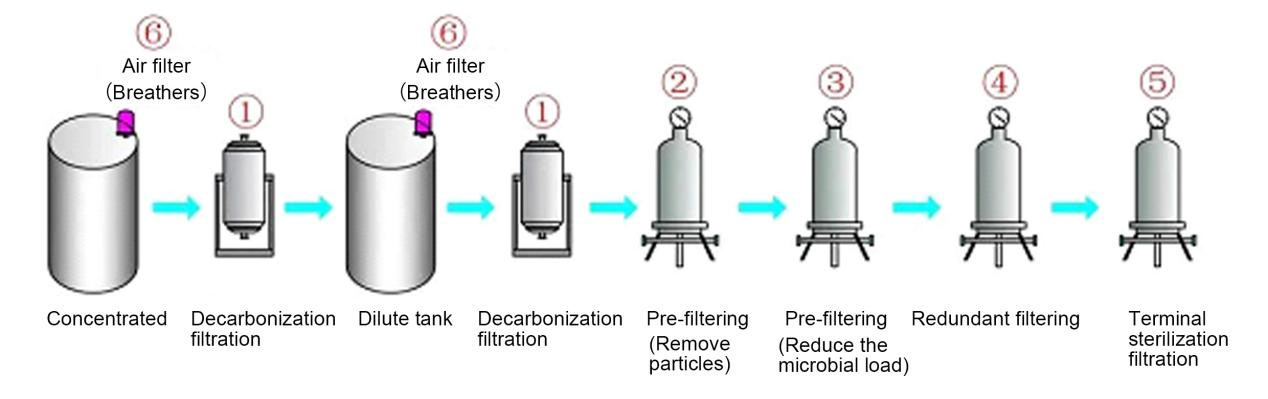
Filtration is widely used in different process stages of large and small volume injections. It mainly includes decarbonization filtration, particle removal and pre-filtration to reduce microbial load, gas sterilization filtration, redundant filtration and sterilization filtration of end products. etc. The main filtering sites are:
pleated filter cartridge factory
1. Decarbonization filtration: remove activated carbon particles and colloidal impurities, extending the service life of downstream filters
2. Pre-filtration (particle removal): further remove activated carbon particles and colloidal impurities, extending the service life of downstream filters
3. Pre-filtration (reduce microbial load): Reduce microbial load and extend the service life of downstream sterilization filter elements
4. Redundant filtration: remove bacteria and microorganisms in the filtrate
5. Terminal sterilization filtration: remove bacteria and microorganisms in the terminal filtrate
6. Respirator: balance tank pressure and prevent pollution
Common large and small volume injection production processes
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN