News
Home News Technical articles In double-pass sterilizing filtration, is the redundant filter the first or second pass?
Home News Technical articles In double-pass sterilizing filtration, is the redundant filter the first or second pass?
In double-pass sterilizing filtration, is the redundant filter the first or second pass?
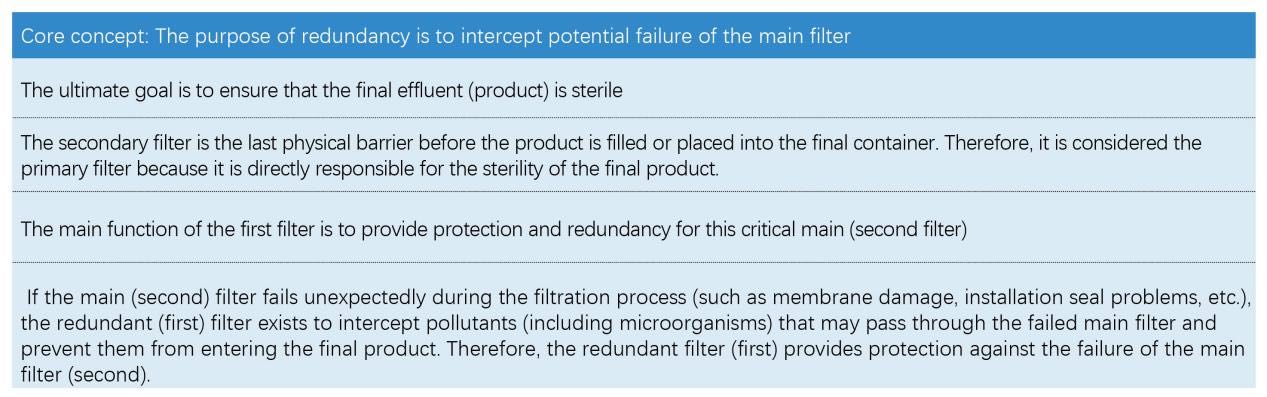
2025-07-24 Eternalwater In a double-pass sterilization filtration system (common in industries with strict aseptic requirements such as pharmaceuticals, biological products, food and beverages), the first filter is called a "redundant filter" and the second is called a "primary filter", mainly based on risk control, fault detection logic and industry standard practices. This is consistent with the core meaning of the word "redundancy" in engineering - providing backup for critical systems in case the main system fails.

Figure 1
Detailed explanation as below:
1.
2.Logical sequence of fault detection:
● Ideal situation: Both filters pass the test, proving that the filtration process is effective.
● The most critical failure scenario: The primary (secondary) filter integrity test fails. This directly means that product sterility may be compromised because it is the last barrier. At this point, regardless of whether the redundant filter passes the test, the batch of product will usually be judged as unqualified because the primary barrier has failed.
● Scenario where redundancy protection works: The redundant (first) filter integrity test fails, while the main (second) filter passes the test. This means that the main filter fulfills its duties, maintains integrity during the filtration process, and ensures the sterility of the final product. Although the redundant filter fails, it fails before the main filter, and the main filter successfully intercepts all contaminants that may pass through the failed redundant filter.
Therefore, the batch can usually be accepted (of course after rigorous investigation and evaluation). This is the purpose and value of redundant design - when the main filter is working properly, the failure of the redundant filter (if it occurs) will not jeopardize the sterility of the final product.
3.Why not a second one as redundancy?
● If the second filter is defined as "redundant", it means that the first filter is the "primary filter"
● If the primary (first) filter fails (which cannot be detected in real time during the filtration process), the contaminants will flow directly to the "redundant" (secondary) filter.
● If the "redundant" (secondary) filter works properly, it can indeed intercept pollutants and ensure the sterility of the final product. From the interception effect, it seems that the purpose of redundancy has been achieved.
However, the key issue is fault detection and batch release: after filtration, if the "redundant" (second pass) filter passes the integrity test, but the "primary" (first pass) filter fails, can we release the product? Not easily! Because the failure of the "primary" (first pass) filter means that the "redundant" (second pass) filter has been challenged far beyond its design load (all the microorganisms and particles that should have been intercepted by the first pass have rushed to the second pass). Although the second pass test passed, it may have experienced: increased risk of microbial penetration: a large number of microbial impacts may exceed its retention capacity limit.
Particle blockage/masking: a large number of particles may block the membrane pores or form filter cakes. Although the membrane itself is not broken during the test (the bubble point test is passed), the microorganisms may be "entrained" through or exist in the blocked area without being detected. Not simulating the real load: The integrity test is performed under clean and no-load conditions, which cannot fully simulate the actual state of filtering high-load pollutants. Therefore, in the case of failure of the "main" (first) filter, even if the "redundant" (second) filter passes the test, the sterility risk of the batch of products is considered to be significantly increased and is generally unacceptable. This greatly reduces the value of the second filter as "redundancy" in batch release decisions, and fails to effectively achieve the core redundancy goal of "ensuring product quality when the main filter fails."

4.Industry standards and regulatory requirements
● Major pharmaceutical manufacturing quality management practices (such as GMP), industry guidelines (such as FDA's "Guide for Aseptic Processing of Sterile Drug Products", PDA technical reports), and filter manufacturers' validation requirements all clearly define the filter closest to the final product as the primary filter and the filter upstream of it as the redundant filter.
● This naming and design logic has been widely accepted and enforced by regulators and industries around the world
Summarize:
Naming based on core function: The core function of the "redundant" filter (first stage) is to provide failure protection for the "primary" filter (second stage, last barrier). Risk control focus: The ultimate responsibility for sterility assurance falls on the last barrier (primary filter). The redundant filter is its "insurance". Batch release logic: Redundant (first stage) failure + primary (second stage) intact = product is generally acceptable (redundancy works). Primary (second stage) failure = product failure (regardless of redundancy status). This logic is clear and the risk is controllable. Prevent overload risk: If the primary (first stage) fails, even if the redundant (second stage) test passes, its actual retention capacity may be impaired under overload, and the product risk is high and unacceptable. This proves the necessity of defining the second stage as the main/last barrier. Compliance with regulatory standards: Industry specifications and regulatory requirements clearly support this definition and design.
Therefore, in a standard two-pass sterilizing filtration system, "redundancy" refers to the first filter, which serves as a dedicated backup for the second (primary) filter. The second filter is called "primary" because it bears the direct responsibility for ensuring the sterility of the final product. The role of the redundant filter is to ensure that the final product is still safe in the event of failure of the primary filter.
Original from:deepseek
Latest News
Read more
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
CopyRight © Hangzhou Eternalwater Filtration Equipment Co., Ltd. 2002-2025
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN