What is the difference between 0.22μm and 0.2μm ?
2025-07-31 Eternalwater The difference between 0.22μm and 0.2μm primarily lies in naming conventions and industry applications, as both actually represent the same pore size grade and belong to the sterilizing-grade filtration category.In the field of sterile filtration, 0.22μm and 0.2μm are two common nominal pore size ratings for sterilizing-grade filters. Although the numerical difference is minimal (only 0.02μm), their distinctions are mainly reflected in four aspects: historical evolution, standard definitions, retention capabilities and application validation.
1.Historical Evolution and Naming Origins
|
Pore Size |
Background Description |
|
0.22μm |
The early industry habitual terminology originated in the 1970s. At that time, filtration technology was not mature, with actual filtration precision approximating 0.22μm, leading to its widespread adoption. |
|
0.2μm |
Modern standardized nomenclature, driven by international standards organizations (such as ASTM, ISO) and regulatory agencies (FDA, EU GMP), aims to unify precision requirements. |
|
Key Points:0.22μm is essentially the "old name" of 0.2μm. The current international standard for sterilization-grade filters has been unified to 0.2μm (such as ASTM F838-05), but some companies still use the "0.22μm" label due to historical habits, and the actual product performance is consistent with 0.2μm |
|
2.Key Differences in Retention Capability
Although the values are close, 0.2μm filters demonstrate superior retention for specific microorganisms, particularly the following challenge organisms:
|
Microorganisms |
Minimum size |
0.22μm filter |
0.2μm filter |
|
(Brevundimonas diminuta) |
0.3–0.4μm |
Potential breakthrough (old standard) |
Complete retention(LRV≥7) |
|
Mycoplasma |
0.2–0.3μm |
Higher risk |
Highly efficient retention |
|
LRV(Log Reduction Value): Modern 0.2μm filters achieve LRV ≥7 for B. diminuta (i.e., ≤1 organism may pass per 10⁷ challenged), while traditional 0.22μm filters may only reach LRV ≥6. |
|||
3.Standards and Validation Methods
|
Project |
0.22μm(old standard) |
0.2μm(current standard) |
|
Testing Standards |
No unified method |
ASTM F838-05(mandatory use of B. diminuta challenge) |
|
Validation Resssquirements |
Dependent on manufacturer-defined parameters |
Requires 3 batches of bacterial challenge, each batch ≥10⁷ cfu/cm² |
|
Pore Size Definition |
Nominal |
Absolute |
|
Industry Consensus: Only filters compliant with ASTM F838-05 qualify as "sterilizing-grade", with a nominal 0.2μm pore size rating. Filters with a nominal 0.22 μm cannot guarantee the sterilizing capacity if they are not validated by this standard. ASTM F838-05(2013)--Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration, published in 2005 by the American Society for Testing and Materials (US-ASTM)
|
||
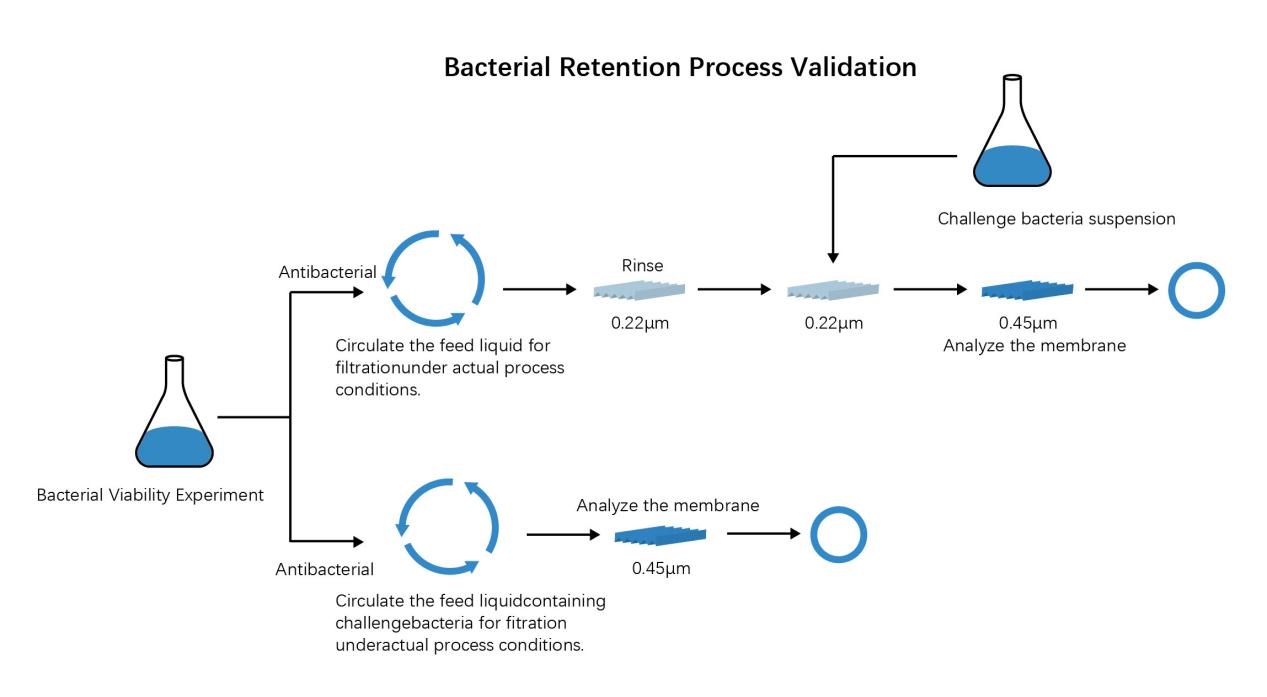
4.Practical Application Selection
|
Scenarios |
Recommended Selection |
Reasons |
|
|
Injections, vaccines, and other sterile preparations |
0.2μm |
Compliant with GMP and pharmacopeia mandatory standards (e.g., USP <71>) |
|
|
Non-critical buffers, gas filtration |
0.22μmor 0.2μm |
lower risk and cost-effective |
|
|
Bioreactor air intake, cell culture |
0.2μm |
Effective retention of small microorganisms like mycoplasma
|
|
Summary:Core Differences
|
Comparison Dimension |
0.22μm |
0.2μm |
|
Essence |
Traditional habitual terminology |
International standard defined pore size |
|
Retention Capacibility |
May be insufficient (especially for B. diminuta ) |
Strictly guarantee LRV≥7 performance |
|
Standard Compliance |
May not fully comply with ASTM F838-05 |
ASTM F838-05 Mandatory ASTM F838-05 compliance |
|
Regulatory Acceptance |
May face regulatory scrutiny |
Globally recognized standard |
|
Practical Suggestions: Priority should be given to the filters with a nominal 0.2 μm and validated ASTM F838-05, regardless of whether they are labeled as "0.22μm". During actual procurement, request the bacterial challenge test report and integrity test limits for the filter rather than only focusing on the pore size value. |
||
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN