Sterilizing grade capsule filter 2 inch / 4 inch KMT Series
Eternalwater KMT series--of capsule filter products cover a variety of specifications and materials and can be applied to bioprocess filtration steps. They meet the requirements of applications from small-scale experiments to large-scale production, with superior filtration performance and reliable sterilization protection.
- Industry Application
- Bacterial removal filtration for large and small volume infusions
Bacterial removal filtration for Vaccines, Biologicals and Antibiotics
Bacterial removal filtration for serum and plasma separation
Bacterial removal filtration for cell culture media
Bacterial removal filtration for diagnostic reagents and preparations containing proteins, vitamins and preservatives
Disposable stock solution sterile filtration
Buffer solution filtration
- DOWNLOAD
- INQUIRY NOW
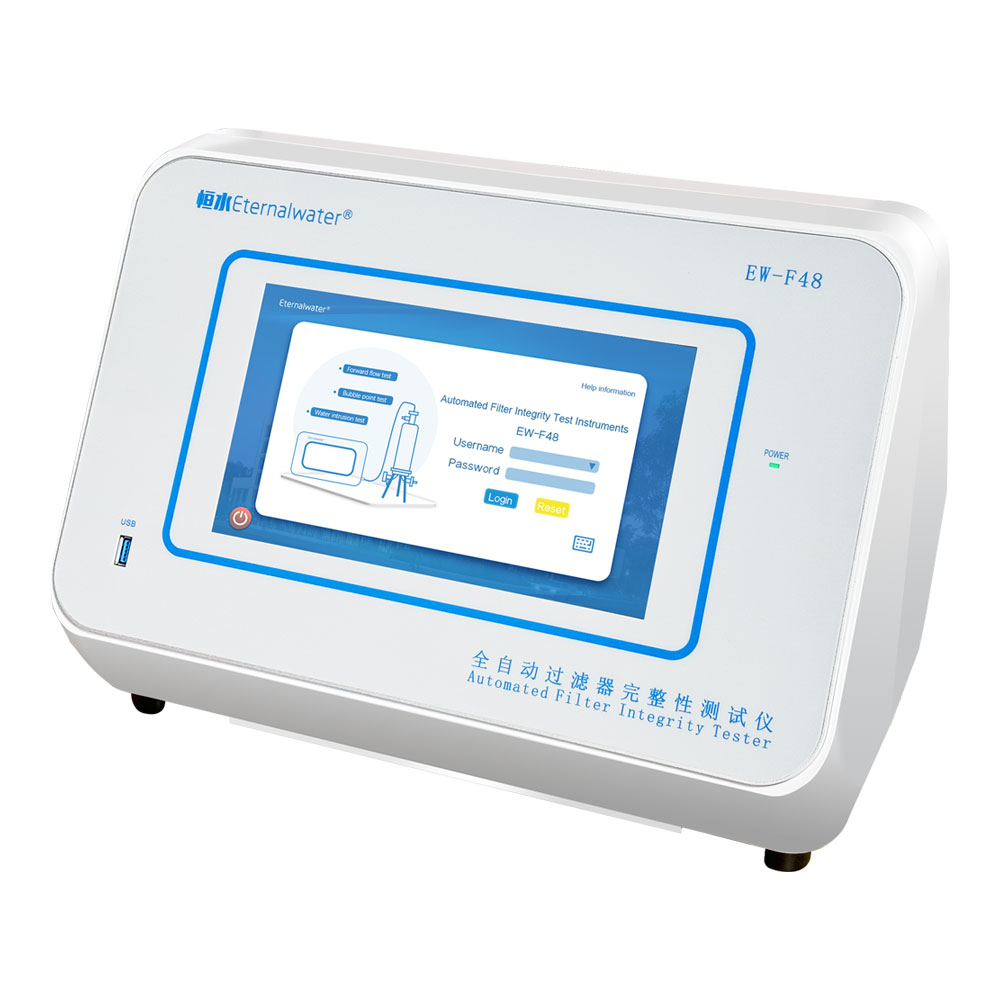
PRODUCT DESCRIPTION
- Nominal size:
- 2inch | 4inch
- Outer diameter:
- 71mm
- Filtration area:
- 1200cm2 | 2400cm2
- Housing material:
- Polypropylene
- Center pole material:
- Polypropylene
- Support layer material:
- Polypropylene
- Vent O-ring Material:
- Silicone
- Vent /Drain Outlet Diameter:
- Φ4mm
- Maximum Operating Pressure Difference:
- 4 bar (58 psi) at 25°C
- Housing pressure:
- 5.5 bar (80 psi) at 25°C
- Sterilization:
- Can be sterilized by Gamma radiation up to 45KGy Can be sterilized by high-pressure steam at 126°C for 30 minutes three times (in-line steam sterilization is not available)
- Bacterial Endotoxin:
- According to the bacterial endotoxin test method stipulated in the general rule (1143) of the Chinese Pharmacopoeia 2020 edition. As determined by Limulus reagent (LAL), the aqueous extract content is < 0.25 EU/mL
- Microbial Challenge Test:
- >107cfu/cm2; according to ASTM® test method passed Pseudomonas diminuta (B. diminuta) ( ATCC® 19146)
- Biosecurity:
- All materials meet USP <88> Reaction Test Standards for Plastic Materials Class VI-70°C
- TOC/Conductivity:
- TOC value≤0.5mg/L, conductivity value≤1.3us/cm. According to the Chinese Pharmacopoeia 2020 Edition, the general rule (0682) the determination method of total organic carbon in pharmaceutical water and the general rule (0681) the determination method of the conductivity of pharmaceutical water
- Cleanliness:
- According to the light inspection method stipulated in the general rules (0904) of the Chinese Pharmacopoeia 2020 edition, no fibers and other visible foreign matter were detected in the filtrate
- Integrity Test:
- Bubble point, diffusion flow, water intrusion; please consult the sales engineer of Eternalwater Company for detailed parameters
Ordering Information
related products
- Industry Application
- Life Sciences
- water treatment
- Industrial Filtration
- Food & Beverage
- Microelectronics
- Laboratory
- New energy battery
- Contact Us
- [email protected]
- +86-571-87022016
- +86-571-87293027
- +8613675899519
- Subscribe for Join Us!
- Join us and get detail information,technical parameter and new products etc.
CopyRight © Hangzhou Eternalwater Filtration Equipment Co., Ltd. 2002-2025
- [email protected]
- Jenny wu
- +8613675899519
- +86(571) 87022016

 EN
EN  ES
ES AR
AR JP
JP CN
CN